Botox® is a non-surgical treatment that improves age-related wrinkles and frown lines. The simple treatment involves small injections to relax the tensed muscles and overlying wrinkled skin. Results typically last three to four months.
Botox® $10 Thursdays!
Every third Thursday, Botox® is only $10 a unit!
(Regular cost is $12 a unit. To receive the discount on Botox® units, an appointment must be done on the third Thursday of the month.)
Benefits:
- Non-surgical treatment
- Smooths and prevents wrinkles
- Slows the effects of aging
- Non-invasive and fast treatments
- Inexpensive
- Customizable treatment options
Ready to schedule a free consultation?
- Message us through the secure Klara chat below
- Text us at 308-280-2295
- Give us a call at 308-995-4431
Before and After Photos:
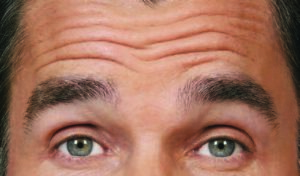
Before:
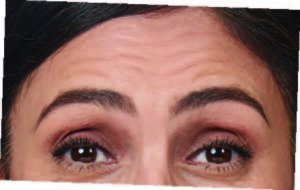
After (Day 30):
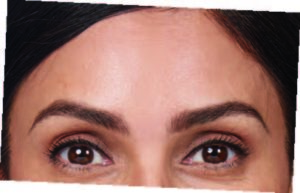
Actual patient. Results may vary. By prescription only.
Photos taken at maximum eyebrow elevation before and 30 days after treatment with BOTOX® Cosmetic. In 2 clinical studies of healthy
adults, 61% and 46% had a ≥ 2-grade improvement at day 30.1,*
*Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.
Before:

After (Day 30):

Actual patient. Results may vary. By prescription only.
Photos taken at full smile before and 30 days after treatment with BOTOX® Cosmetic. In 2 clinical studies, 26.1% and 20.3% of adults
had a ≥ 2-grade improvement at day 30. In one of these studies, 67.9% had mild or no crow’s feet lines at day 30 after treatment.1,*
*Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.
Before:
After (Day 30):
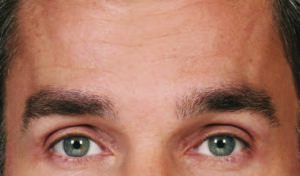
Actual patient. Results may vary. By prescription only.
Photos taken at maximum eyebrow elevation before and 30 days after treatment with BOTOX® Cosmetic. In 2 clinical studies of healthy
adults, 61% and 46% had a ≥ 2-grade improvement at day 30.1,*
* Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.
Before:

After (Day 30):

Actual patient. Results may vary. By prescription only.
Photos taken at full smile before and 30 days after treatment with BOTOX® Cosmetic. In 2 clinical studies, 26.1% and 20.3% of adults
had a ≥ 2-grade improvement at day 30. In one of these studies, 67.9% had mild or no crow’s feet lines at day 30 after treatment.1,*
* Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.
Before:
After (Day 7):

Actual patient. Results may vary. By prescription only.
Photos taken at full smile before and 7 days after treatment with BOTOX® Cosmetic. In 2 clinical studies, 26.1% and 20.3% of adults had
a ≥ 2-grade improvement at day 30. In one of these studies, 67.9% had mild or no crow’s feet lines at day 30 after treatment.1,*
* Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.
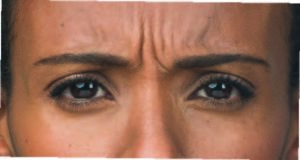
Before:
After (Day 7):
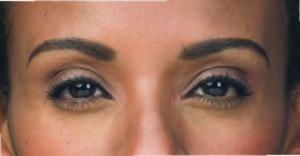
Actual patient. Results may vary. By prescription only.
Photos taken at maximum frown before and 7 days after treatment with BOTOX® Cosmetic. In clinical studies, physicians assessed 74%
of adults had significant improvement at day 7; and 80% had significant improvement at day 30.1,*
* Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.
Frequently Asked Questions:
Botox® is a non-surgical treatment that helps improve moderate to severe wrinkles and frown lines in the forehead, and brow area. The simple treatment is done through small injections into the muscle to relax the tensed muscles. Results typically last three to four months.
Botox® is approved for clients who are 18 years and older. Most experts agree that clients in their mid to late 20s and early 30s are at a good age for preventative treatments.
The results for Botox® vary depending on the client. Typical results for Botox® can last up to 3-4 months.
At your free consultation, our certified provider will discuss your areas of concern, explain the procedure, and make dosing recommendations.
Injections can be administered during the same visit or at a future visit.
Your appointment will last for about 20-30 minutes, and the injections take about 10 minutes of that time. Your provider will clean your face to remove prior to injections.
We recommend staying upright for 4 hours after the procedure, not performing any moderate to excessive workouts, not touching areas that were treated, and not washing/scrubbing the face. Your provider will discuss all the steps with you and answer all of your questions at your appointment.
Yes, Botox®, in addition to reducing the appearance of fine lines and wrinkles, can be used to treat conditions such as:
- Chronic Migraines
- Occipital Neurolgia
- Limp Spasticity
- Cervical Dystoria Bruxtism
- Postherpetic Neuralgia (Shingles Rash Pain)
- Temporomandibular Joint (TMJ) Pain (jaw pain & teeth grinding)
- Hyperhidrosis (Excessive Sweating)
- Hypersalivation (Excessive Drooling)
Contact us to learn more about how insurance may be able to help cover the use of Botox® for the above treatments.
Botox® is a non-surgical treatment that helps temporarily improve moderate to severe wrinkles and frown lines in the forehead, eyes, and brow areas by relaxing the muscles that create lines. The results can last up to 3-4 months.
Juvéderm® is a filling agent that adds volume to areas of the face, such as the lips, chin, nasolabial folds, and cheeks. The results vary by product. For example, Juvéderm® Voluma™ XC can last up to one year in the chin with optimal treatment.
At your free consultation, our certified providers will work with you to determine the best solution to help you achieve the results you are looking for.
Yes! Both Botox® and Juvéderm® can be used together as long as they are being injected into separate regions of the face. We can even schedule both injectable treatments to be performed at the same time.
To schedule an appointment, you can message us through the secure chat below, text our clinic at 308-280-2295, or give our clinic a call at 308-995-4431 and ask to set up a consultation!
Ready to schedule a free consultation?
- Message us through the secure Klara chat below
- Text us at 308-280-2295
- Give us a call at 308-995-4431
Above are some examples of patients before and after their Botox® treatments.
© 2021 AbbVie. All rights reserved. BOTOX® and its design are registered trademarks of Allergan, Inc., an AbbVie company.
hcp.BotoxCosmetic.com 1-800-BOTOXMD BCT114098-v3 08/20 007370
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
Indications
BOTOX® Cosmetic (onabotulinumtoxinA) is indicated in adult patients for the temporary improvement in the appearance of: – Moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity – Moderate to severe lateral canthal lines associated with orbicularis oculi activity – Moderate to severe forehead lines associated with frontalis activity
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX® Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses and approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and upper limb spasticity and at lower doses.
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability Between Botulinum Toxin Products The potency units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX® Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method.
Spread of Toxin Effect Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), and 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines) have been reported. Patients or caregivers should be advised to seek immediate medical care if swallowing, speech, or respiratory disorders occur.
Serious Adverse Reactions With Unapproved Use
Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, with some adverse reactions associated with fatal outcomes, have been reported in patients who received BOTOX® injections for unapproved uses. In these cases, the adverse reactions were not necessarily related to distant spread of toxin, but may have resulted from the administration of BOTOX® to the site of injection and/or adjacent structures. In several of the cases, patients had pre-existing dysphagia or other significant disabilities. There is insufficient information to identify factors associated with an increased risk for adverse reactions associated with the unapproved uses of BOTOX®. The safety and effectiveness of BOTOX® for unapproved uses have not been established.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX® of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
Increased Risk of Clinically Significant Effects With Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including generalized muscle weakness, diplopia, ptosis, dysphonia, dysarthria, severe dysphagia, and respiratory compromise from onabotulinumtoxinA (see Warnings and Precautions).
Dysphagia and Breathing Difficulties
Treatment with BOTOX® (onabotulinumtoxinA) and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or oropharyngeal muscles that control swallowing or breathing (see Boxed Warning).
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX® Cosmetic treatment is used in the presence of inflammation at the proposed injection site(s) or when excessive weakness or atrophy is present in the target muscle(s).
Dry Eye in Patients Treated With BOTOX® Cosmetic
There have been reports of dry eye associated with BOTOX® Cosmetic injection in or near the orbicularis oculi muscle. If symptoms of dry eye (eg, eye irritation, photophobia, or visual changes) persist, consider referring patients to an ophthalmologist.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral disease and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
ADVERSE REACTIONS
The most frequently reported adverse reactions following injection of BOTOX® Cosmetic for glabellar lines were eyelid ptosis (3%), facial pain (1%), facial paresis (1%), and muscular weakness (1%).
The most frequently reported adverse reaction following injection of BOTOX® Cosmetic for lateral canthal lines was eyelid edema (1%).
The most frequently reported adverse reactions following injection of BOTOX® Cosmetic for forehead lines with glabellar lines were headache (9%), brow ptosis (2%), and eyelid ptosis (2%).
DRUG INTERACTIONS
Co-administration of BOTOX® Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like compounds) should only be performed with caution as the effect of the toxin may be potentiated. Use of anticholinergic drugs after administration of BOTOX® Cosmetic may potentiate systemic anticholinergic effects.
The effect of administering different botulinum neurotoxin products at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of BOTOX® Cosmetic.
USE IN SPECIFIC POPULATIONS
There are no studies or adequate data from postmarketing surveillance on the developmental risk associated with use of BOTOX® Cosmetic in pregnant women. There are no data on the presence of BOTOX® Cosmetic in human or animal milk, the effects on the breastfed child, or the effects on milk production.
Please see accompanying BOTOX® Cosmetic full Prescribing Information including Boxed Warning and Medication Guide.